
Judit Ovádi
Hungarian Academy of Sciences, Hungary
Title: Challenging drug target for moonlighting and chameleon proteins
Biography
Biography: Judit Ovádi
Abstract
The conformational diseases such as Parkinson’s disease (PD) and Multiple System Atrophy (MSA) represent an important group of neurodegenerative disorders. The hallmarks of these diseases are the a-synuclein (SYN) and the recently discovered Tubulin Polymerization Promoting Protein (TPPP/p25). Both proteins are disordered with chameleon characteristics and expressed distinctly in neurons and oligodendrocytes (OLGs), respectively; notwithstanding they are co-enriched and co-localized in pathological inclusions in the case of PD and MSA. TPPP/p25 is the prototype of the Neomorphic Moonlighting Proteins by displaying both physiological and pathological functions due to their interactions with distinct partners. At physiological conditions TPPP/p25 modulates the dynamics and stability of the microtubule system; its expression is crucial for the differentiation of OLGs, the major constituents of the myelin sheath. The assembly of TPPP/p25 and SYN, as fatal initiative, of the etiology of PD and MSA has been established. Due to the unique structural and functional features of TPPP/p25, a new innovative strategy has to be evaluated to inhibit and/or destruct specifically the interaction of TPPP/p25 with SYN; this could be fulfill by targeting of the interface of the pathological complex without affecting the physiological one. Our studies underline that targeting multifunctional proteins is a challenging task; nevertheless, the validation of a drug target can be achieved by identifying the interface of complexes of the partner proteins existing at the given pathological conditions.
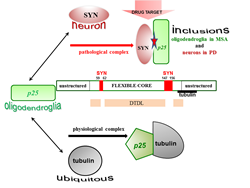
Figure 1: Distinct interfaces of TPPP/p25 are involved in its hetero-association with tubulin and SYN as physiological and pathological partners. The interaction of the disordered TPPP/p25 with tubulin results in significant conformational changes; this effect does not manifest itself in the case of its association with SYN. Although the deletions within the middle CORE segments do not abolish the binding of TPPP/p25 mutants to SYN, except in the case of the double truncated double loop mutant (DTDL), due to its chameleon feature; however, a potential segments (marked red) were identified as potential drug target.
