
Ida Chiara Guerrera
INSERM, France
Title: Proteomics of rare genetic diseases: Impact of cystinosin mutations on protein stability and protein network
Biography
Biography: Ida Chiara Guerrera
Abstract
Statement of the Problem: Cystinosis is a rare autosomal recessive storage disorder characterized by defective lysosomal efflux of cystine due to mutations in the CTNS gene encoding the lysosomal cystine transporter, cystinosin. Over 100 mutations have been reported, leading to varying disease severity, often in correlation with residual cystinosin activity as a transporter. However, the correlation between genotype and phenotype is not always clear and we applied different proteomics approaches to better understand the mechanisms of this disease. To unveil additional roles of cystinosin, we studied the protein interaction of the WT cystinosin and different mutations. Furthermore, we focused on an atypical mutation concerning protein glycolsilation, ΔITILELP, that sometimes leads to severe forms.
Methodology & Theoretical Orientation: Co-immunoprecipitation and dynamic SILAC were used. All analyses were performed on a nanoRSLC Q-Exactive Plus MS.
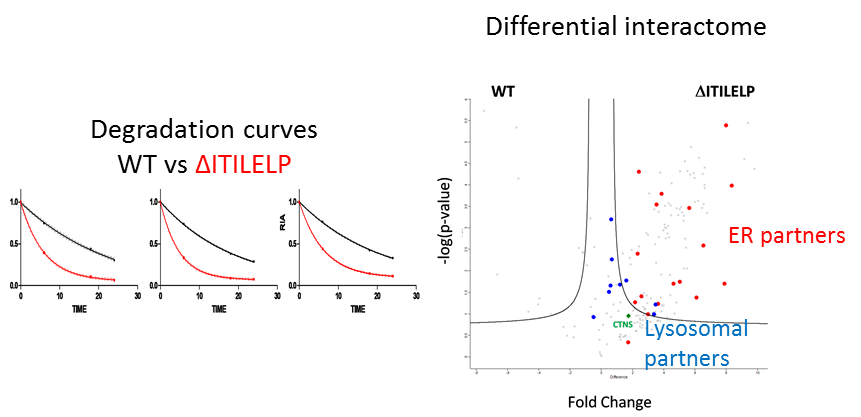
Findings: We found cystinosin interacts with almost all components of vacuolar H(+)-ATPase and the Ragulator complex and with the small GTPases Ras-related GTP-binding protein A (RagA) and RagC. These interactions are lost when cystinosin carries severe loss-of-activity mutations. We also showed that wild-type cystinosin is very stable, while ΔITILELP is degraded three times more rapidly. We observed that in the lysosome, ΔITILELP is still capable of interacting with the V-ATPase complex and some members of the mTOR pathway, similar to the wild-type protein. Our interactomic and immunofluorescence studies showed that ΔITILELP is partially retained at the endoplasmic reticulum (ER).
Conclusion & Significance: Our results show a dual role for cystinosin as a cystine transporter and as a component of the mTORC1 pathway, and provide an explanation for the appearance of Fanconi syndrome in cystinosis. We also found that the high turnover of ΔITILELP, due to its immature glycosylation state in combination with low transport activity, might be responsible for the phenotype observed in some patients.
Recent Publications
1. Nevo N (2017) Impact of cystinosin mutations on protein stability by differential dynamic SILAC. Molecular and Cellular Proteomics (In press).
2. Lipecka J (2016) Sensitivity of mass spectrometry analysis depends on the shape of the filtration unit used for filter aided sample preparation (FASP). Proteomics. 16(13):1852-7.
3. Chhuon C (2016) Changes in lipid raft proteome upon TNF-α stimulation of cystic fibrosis cells. J. Proteomics. 145:246-53.
4. Andrzejewska Z (2016) Cystinosin is a component of the vacuolar H+-ATPase-Ragulator-Rag complex controlling mammalian target of rapamycin complex 1 signaling. J Am Soc Nephrol. 27(6):1678-88.
5. Ramond E (2015) Importance of host cell arginine uptake in Francisella phagosomal escape and ribosomal protein amounts. Mol Cell Proteomics. 14(4):870-81.
