
K. Venkateswara Swamy
Dr. D Y Patil Biotechnology and Bioinformatics Institute, India
Title: Molecular modeling, docking, dynamics and simulation of deguelin and its derivatives with cyclin D1 and cyclin E in cancer cell signaling pathway
Biography
Biography: K. Venkateswara Swamy
Abstract
Deguelin is a major active ingredient and principal component in several plants, Derris trifoliata Lour. (Leguminosae), Mundulea sericea (Leguminosae), Tephrosia vogelii Hook.f. (Leguminosae) and potential molecule to target cancer cell signaling pathway proteins. As a complex natural extract, deguelin interacts with various molecular targets to exert its anti-tumor properties at nanomolar levels. Deguelin induced cell apoptosis by blocking anti-apoptotic pathways, while inhibiting tumor cell propagation and malignant transformation through p27-cyclin-E-pRb-E2F1- cell cycle control and HIF-1αVEGF antiangiogenic pathways. Our research explores the deguelin and its derivatives interaction with crystal structure of cyclin D1 (PDB ID: 2W96) and cyclin E (PDB ID: 2AST) to understand the better molecular insights. Molecular modelling of ligands (deguelin and its derivatives) were carried out by Avogadro software till stable confirmation obtained. The partial charges for the ligands were assigned as per standard protocol for molecular docking. All docking simulation were performed with AutoDock Vina on workstation. Virtual screening was done for all docked molecules based on binding energy and hydrogen bonding affinity. Molecular dynamics (MD) and Simulation (10 ns and 12 ns for cyclin D1 and cyclin E1 respectively) was done using GROMACS 5.1.1 software to explore the interaction stability. All the stable confirmations for cyclin D1 and cyclin E proteins trajectories was captured at various time intervals. Few compounds screened based on high affinity as inhibitors for cyclin D1 and cyclin E and may inhibit the cell cycle in cancer cell signaling under in vitro and in vivo experiments.
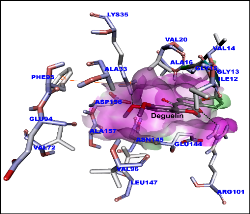
Figure 1: Molecular docking of deguelin with cyclin D1 protein. Surface shown in pink. Amino acids and deguelin hydrogen bond interaction with dotted lines.
Recent Publications
1. Prasad Dandawate, Kiranmayi Vemuri, K Venkateswara Swamy, Ejazuddin M Khan, Manjula Sritharan and Subhash Padhye (2014) Synthesis, characterization, molecular docking and anti-tubercular activity of Plumbagin-Isoniazid Analog and its β-cyclodextrin conjugate. Bioorganic & Medicinal Chemistry Letters 24(21):5070-5075.
2. Prasad Dandawate, Aamir Ahmad, Jyoti Deshpande, K Venkateswara Swamy, Ejazuddin M Khan, Madhukar Khetmalas, Subhash Padhye and Fazlul Sarkar (2014) Anticancer phytochemical analogs 37: Synthesis, characterization, molecular docking and cytotoxicity of novel plumbagin hydrazones against breast cancer cells. Bioorganic & Medicinal Chemistry Letters, 24:2900-2904.
3. P Sharma, P Patil, N Rao, K V Swamy, M B Khetmalas and G D Tandon (2014) Mapping biodiversity of indigenous freshwater chlorophytes. Research Journal of Pharmaceutical, Biological and Chemical Sciences 5(3):1632-1639.
4. Shibnath Ghataka, Alok Vyas, Suniti Misra, Paul O’Briend, Ajit Zambre, Victor M Fresco, Roger R Markwald, K Venkateshwara Swamy, Zahra Afrasiabif, Amitava Choudhury, Madhukar Khetmalas and Subhash Padhye (2013) Novel di-tertiary-butyl phenylhydrazones as dual cyclooxygenase-2/5-lipoxygenase inhibitors: synthesis, COX/LOX inhibition, molecular modeling, and insights into their cytotoxicities. Bioorganic & Medicinal Chemistry Letters 24:317-324.
5. Siddiqui A, Dandawate P, Rub R, Padhye S, Aphale S, Moghe A, Jagyasi A, Venkateswara Swamy K, Singh B, Chatterjee A, Ronghe A and Bhat H K (2013) Novel Aza-resveratrol analogs: Synthesis, characterization and anticancer activity against breast cancer cell lines. Bioorganic & Medicinal Chemistry Letters 23:635–640.


