Tamas Hegedus
Semmelweis University, Hungary
Title: In silico modeling of ABCG2/BCRP structure and dynamics sheds light on xenobiotic recognition and transport function
Biography
Biography: Tamas Hegedus
Abstract
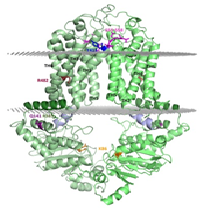
ABCG2 is an ATP binding cassette transporter protein, containing a nucleotide binding domain and a transmembrane region. It functions as a homo-dimer multidrug resistance (MDR) transporter in the plasma membrane, extruding a wide range of molecules from the cell. ABCG2 affects the pharmacokinetics of various drugs and protects the stem cells and cancer (stem) cells from xenobiotic and chemotherapeutic agents. This transporter is present in key pharmacological barriers and drug metabolizing organs. However, ABCG2 is also involved in the transport of endobiotics, e.g. porphyrins and uric acid. Its Q141K variant exhibits decreased functional expression leading to increased drug accumulation and decreased urate secretion. To overcome MDR and correct ABCG2 related pathological phenomena, learning its structural and dynamic properties is a major objective in the field. Still, there has been no reliable molecular model available for this protein, as the published structures of other ABC transporters could not be properly fitted to the ABCG2 topology and experimental data. The recently published high resolution structure of a close homologue, the ABCG5/ABCG8 heterodimer, revealed a new ABC transporter fold, unique for ABCG proteins. We generated a structural model of the ABCG2 homo-dimer based on this fold and confirmed its validity using experimental data. We also performed molecular dynamics simulations and in silico docking to understand the effect of mutations including R482G and Q141K and the mechanism of substrate recognition. The ABCG2 model, we present may have significant impact on understanding drug distribution and toxicity as well as drug development against cancer chemotherapy resistance or gout.